Cationic red-emitting probes for the rapid and selective detection of SO2 derivatives in aqueous and cellular environments†
Abstract
Two water-soluble compounds based on a coumarin–pyridinium scaffold were synthesized for rapid and selective detection of sulfite. The detection strategy is possible through a Michael addition reaction of the sulfite to the cyano-vinyl double bond, distinguishable through the hypsochromic shifts in absorption and emission maxima. The probes exhibited high selectivity for sulfite, with a detection limit of 1.47 μM for dye 1 and 2.8 μM for dye 2 and a near instant response time of 10–20 seconds over other analytes and reactive sulfur species. Importantly, the probes also exhibit a robust colorimetric response in the presence of sulfite. Cellular imaging studies have demonstrated that the probes are non-toxic and suitable for the detection of sulfite levels in cells. Additionally, the probes have demonstrated sensitivity to detect endogenous sulfite levels in hepatic cancer cells.
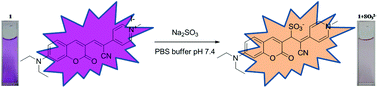


 Please wait while we load your content...
Please wait while we load your content...