Introduction of Mn(iii) to regulate the electronic structure of fluorine-doped nickel hydroxide for efficient water oxidation†
Abstract
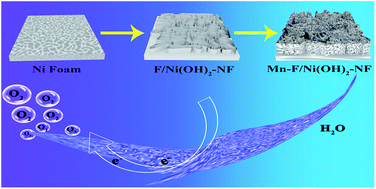
OER is the key step to increase the rate of water-splitting reaction. Design and construction of appropriate defects is an effective strategy to enhance catalytic activity. Mn has stronger e−–e− repulsion by the local influence of its 3d orbital electrons. When Mn(III) was successfully introduced into two dimensional F-doped Ni(OH)2, it can tune the surface electronic structure of the F-doped Ni(OH)2 to increase its oxygen deficiency content. In this work, the as-synthesized Mn and F co-doped Ni(OH)2–NF on Ni foam (Mn–F/Ni(OH)2–NF) shows remarkable oxygen evolution performance, exhibiting 233 mV overpotential at 20 mA cm−2, and the Tafel slope is 56.9 mV dec−1 in 1 M KOH. The performance is better than that of the same loading of IrO2 on Ni foam. Density functional theory (DFT) calculations further show that the introduction of oxygen defects can significantly improve the OER catalytic performance of Mn–F/Ni(OH)2–NF.


 Please wait while we load your content...
Please wait while we load your content...