Insight into the dynamics of APOBEC3G protein in complexes with DNA assessed by high speed AFM†
Abstract
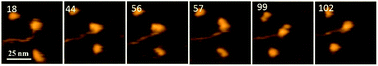
APOBEC3G (A3G) is a single-stranded DNA (ssDNA) binding protein that restricts the HIV virus by deamination of dC to dU during reverse transcription of the viral genome. A3G has two zinc-binding domains: the N-terminal domain (NTD), which efficiently binds ssDNA, and the C-terminal catalytic domain (CTD), which supports deaminase activity of A3G. Until now, structural information on A3G has been lacking, preventing elucidation of the molecular mechanisms underlying its interaction with ssDNA and deaminase activity. We have recently built a computational model for the full-length A3G monomer and validated its structure using data obtained by time-lapse High-Speed Atomic Force Microscopy (HS AFM). Here time-lapse HS AFM is applied to directly visualize the structure and dynamics of A3G in complexes with ssDNA. Our results demonstrate a highly dynamic structure of A3G, where two domains of the protein fluctuate between compact globular and extended dumbbell structures. Quantitative analysis of our data revealed a substantial increase in the number of A3G dumbbell structures in the presence of the DNA substrate, suggesting that the interaction of A3G with the ssDNA substrate stabilizes this dumbbell structure. Based on these data, we proposed a model explaining the interaction of globular and dumbbell structures of A3G with ssDNA and suggested a possible role of the dumbbell structure in A3G function.


 Please wait while we load your content...
Please wait while we load your content...