The supramolecular structure and van der Waals interactions affect the electronic structure of ferrocenyl-alkanethiolate SAMs on gold and silver electrodes†
Abstract
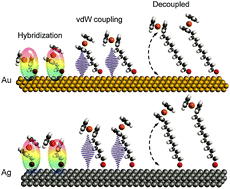
Understanding the influence of structural properties on the electronic structure will pave the way for optimization of charge transport properties of SAM devices. In this study, we systematically investigate the supramolecular and electronic structures of ferrocene (Fc) terminated alkanethiolate (SCnFc) SAMs on both Au and Ag substrates with n = 1–15 by using a combination of synchrotron based near edge X-ray absorption spectroscopy (NEXAFS), photoemission spectroscopy (PES), and density functional theory (DFT) calculations. Odd–even effects in the supramolecular structure persist over the entire range of n = 1–15, which, in turn, explain the odd–even effects in the onset energy of the highest occupied molecular (HOMO) orbital. The orientation of the Fc moieties and the strength of Fc-substrate coupling, which both depend on n, affects the work function (WF). The variation of WF shows an odd–even effect in the weak electrode–Fc coupling regime for n ≥ 8, whereas the odd–even effect diminishes for n < 8 due to hybridization between Fc and the electrode (n < 3) or van der Waals (vdW) interactions between Fc and the electrode (n = 3–7). These results confirm that subtle changes in the supramolecular structure of the SAMs cause significant electronic changes that have a large influence on device properties.


 Please wait while we load your content...
Please wait while we load your content...