Unravelling the physisorption characteristics of H2S molecule on biaxially strained single-layer MoS2†
Abstract
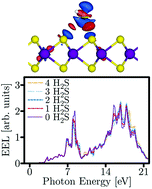
Sensing ultra-low levels of toxic chemicals such as H2S is crucial for many technological applications. In this report, employing density functional theory (DFT) calculations, we shed light on the underlying physical phenomena involved in the adsorption and sensing of the H2S molecule on both pristine and strained single-layer molybdenum disulfide (SL-MoS2) substrates. We demonstrate that the H2S molecule is physisorbed on SL-MoS2 for all values of strain, i.e. from −8% to +8%, with a modest electron transfer, ranging from 0.023e− to 0.062e−, from the molecule to the SL-MoS2. According to our calculations, the electron-donating behaviour of the H2S molecule is halved under compressive strains. Moreover, we calculate the optical properties upon H2S adsorption and reveal the electron energy loss (EEL) spectra for various concentrations of the H2S molecule which may serve as potential probes for detecting H2S molecules in prospective sensing applications based on SL-MoS2.


 Please wait while we load your content...
Please wait while we load your content...