Integrity of zinc finger motifs in PML protein is necessary for inducing its degradation by antimony†
Abstract
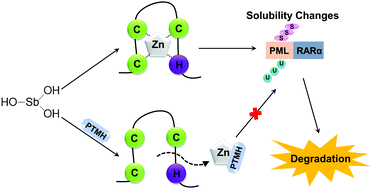
Antimony (Sb) belongs to the same group as arsenic (As) in the periodic table, and both share similar characteristics. However, Sb2O3 (SbIII) has no methylation capacity, unlike arsenic trioxide (As2O3). In the present study, we determined the effect of SbIII on NB4 cells and found that antimony could induce PML-RARα fusion protein degradation, reorganization of PML-NBs, and NB4 cell differentiation with low cytotoxicity. On the other hand, zinc finger motifs in PML protein are considered to be a key target binding site for arsenic-induced PML-RARα protein degradation. Interestingly, antimony and arsenic lost their ability to degrade PML-RARα fusion protein in NB4 cells following pretreatment with phenanthroline (i.e., chelator of zinc ions), indicating that the integrity of zinc finger motifs in PML-RARα fusion protein is a fundamental condition for inducing the protein's degradation by antimony and arsenic. Moreover, we found that SbIII could not induce mutant PML (e.g., A126V and L218P) solubility change and degradation, similar to As2O3. In contrast, we found that the organic antimony compound phenylstibine oxide (PSO) could induce mutant PML protein degradation. In conclusion, our results indicate that SbIII might also be a promising agent to treat acute promyelocytic leukemia, in the same manner as As2O3.


 Please wait while we load your content...
Please wait while we load your content...