Targeting of the mitochondrion by dinuclear thiolato-bridged arene ruthenium complexes in cancer cells and in the apicomplexan parasite Neospora caninum
Abstract
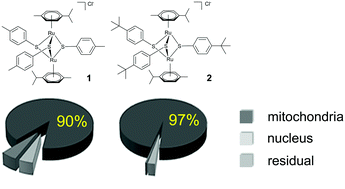
A library of 18 dinuclear-thiolato bridged arene ruthenium complexes, some of which with demonstrated activity against cancer cells, was screened for activity against a transgenic Neospora caninum strain that constitutively expresses beta-galactosidase. Initial assessments were done at concentrations of 2500, 250, 25 and 2.5 nM, and 5 compounds were further evaluated with regard to their half maximal proliferation-inhibiting concentration (IC50). Among those, [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-CH3)3]Cl (1), [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-But)3]Cl (2) and [(η6-p-MeC6H4Pri)2Ru2(μ2-SCH2C6H4-p-But)2(μ2-SC6H4-p-OH)]BF4 (9) inhibited N. caninum proliferation with low C50 values of 15, 5 and 1 nM, respectively, while [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-OH)3]Cl (3) and [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-mco)3]Cl (5, mco = 4-methylcoumarinyl) were less active (IC50 = 280 and 108 nM, respectively). These compounds did not affect human foreskin fibroblast (HFF) host cells at dosages of 5 μM and above, but impaired proliferation of the human ovarian carcinoma cell line A2780 (IC50 values of 130 nM (1), 30 nM (2), 530 nM (3), 7730 nM (5), 130 nM (9)). A2780 cancer cells were treated with complexes 1, 2, and 5, and biodistribution analysis using inductively coupled plasma mass spectrometry (ICP-MS) showed that most of the drugs accumulated in the mitochondrial fractions. Transmission electron microscopy showed that the parasite mitochondrion is the primary target also in N. caninum tachyzoites, but these compounds, when applied at 200 nM for 15 days in vitro, did not act parasiticidal. Complexes 1, 2 and 9 applied orally at 2 and 10 mg kg−1 day−1 during 5 days in a neosporosis mouse model did not reduce parasite load and did not limit parasite dissemination to the central nervous system. In accordance with these results, ICP-MS carried out on different organs of mice orally administrated with complexes 1 and 9, demonstrated that the drugs were readily absorbed, and after 3 and 48 h, were mainly detected in liver and kidney, but were largely absent from the brain. Thus, dinuclear thiolato-bridged arene ruthenium complexes exhibit interesting activities against N. caninum in vitro, but further modifications of these promising molecules are required to improve their bioavailability and pharmacokinetic properties in order to exert a pronounced and selective effect against N. caninum in vivo.
- This article is part of the themed collection: Metallomics Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...