Isatin and its derivatives: a survey of recent syntheses, reactions, and applications
Abstract
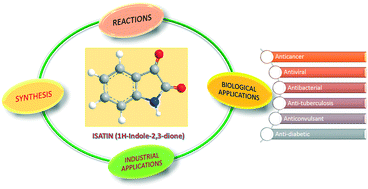
Isatin (1H-indole-2,3-dione) and its derivatives represent an important class of heterocyclic compounds that can be used as precursors for drug synthesis. Since its discovery, a lot of research work has been done regarding the synthesis, chemical properties, and biological and industrial applications of isatin. In this review, we have reported several novel methods for the synthesis of N-, C2-, and C3-substituted and spiro derivatives of isatin. The isatin moiety also shows important chemical reactions such as oxidation, ring expansion, Friedel–Crafts reaction and aldol condensation. These reactions, in turn, produce several biologically viable compounds like 2-oxindoles, tryptanthrin, indirubins, and many more. We have also summarized some recently reported biological activities exhibited by isatin derivatives, like anti-cancer, anti-bacterial, anti-diabetic and others. Special attention has been paid to their anti-cancer activity, and various anti-cancer targets such as histone deacetylase, carbonic anhydrase, tyrosine kinase, and tubulin have been discussed in detail. Other applications of isatin derivatives, such as in the dye industry and in corrosion prevention, have also been discussed.


 Please wait while we load your content...
Please wait while we load your content...