Quantifying the pH shift induced by selective anodic electrochemical reactions in the ion concentration polarization phenomenon†
Abstract
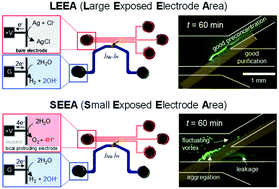
Recently, the ion concentration polarization (ICP) phenomenon has been actively utilized for low abundance biomolecular preconcentration applications. Since ICP significantly rearranges the ion distribution near a permselective membrane, its detailed investigation should be conducted for developing efficient platforms. In particular, proton transport through the membrane critically affects the pH of sample solutions so that continuous monitoring or batch measurement of pH is the priority task to be carried out. Moreover, electrochemical reactions have been overlooked, even though an overpotential is applied to preconcentrate a sample under physiological conditions, and the electrodes are in direct contact with the sample biomolecules. In this work, we experimentally visualized and directly measured how the electrochemical reaction dominated the preconcentration efficiency using two types of electrode configurations; large exposed electrode area (LEEA) and small exposed electrode area (SEEA). Interestingly, significant pH variation was confirmed only in the case of SEEA. As a result, the BSA preconcentration was impeded within a short period in the case of SEEA, but loss-free preconcentration was achieved in the case of LEEA. Therefore, one should pay careful attention to the electrode design of electrokinetic operation, especially when pH-sensitive biomolecules are involved.


 Please wait while we load your content...
Please wait while we load your content...