Electrochemical oxidative cyclization of olefinic carbonyls with diselenides†
Abstract
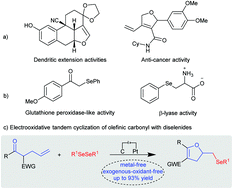
The tandem cyclization of olefinic carbonyls with easily accessible diselenides facilitated by electrochemical oxidation has been successfully developed, which provides an environmentally friendly method for the construction of C–Se and C–O bonds simultaneously. A series of seleno dihydrofurans and seleno oxazolines, bearing fragile heterocycles, subtle C–I bonds and supernumerary vinyl groups, were forged using this elegant chelation strategy. Neither metal catalysts nor external chemical oxidants are required to promote this transformation.


 Please wait while we load your content...
Please wait while we load your content...