Catalytic asymmetric synthesis of chiral phenols in ethanol with recyclable rhodium catalyst†
Abstract
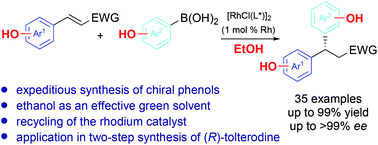
A general method to access diverse chiral phenols by rhodium-catalyzed asymmetric conjugate arylation using hydroxylated arylboronic acids in ethanol was developed. Recycling of the rhodium catalyst by flash chromatography on silica gel was feasible in this system. The synthetic utility of the strategy was demonstrated by efficient synthesis of chiral drug tolterodine.


 Please wait while we load your content...
Please wait while we load your content...