Unsymmetrical triazolyl-naphthyridinyl-pyridine bridged highly active copper complexes supported on reduced graphene oxide and their application in water†
Abstract
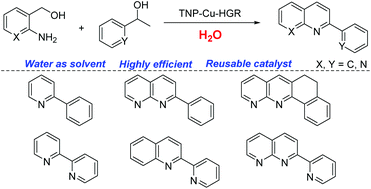
A novel unsymmetrical triazolyl-naphthyridinyl-pyridine ligand was designed and synthesized, and employed in the synthesis of a heterogeneous copper complex on reduced graphene oxide. The resulting copper composite was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and energy dispersive X-ray spectroscopy (EDX). This supported copper catalyst containing unsymmetrical triazolyl-naphthyridinyl-pyridine (only 0.1 mol%) showed excellent catalytic activity in water with good recyclability. Various functionalized quinoline derivatives were successfully synthesized in high yields through the green strategy in water. Other heterocyclic compounds, such as pyridine, 2-(pyridin-2-yl)quinoline, 1,8-naphthyridine, 5,6-dihydronaphtho[1,2-b][1,8]naphthyridine and 2-(pyridin-2-yl)-1,8-naphthyridine derivatives, were achieved in water with more than 80% yields. Mechanism studies revealed that this transformation occurs via dehydrogenation, condensation, and transfer hydrogenation and dehydrogenation processes which was supported by a deuterium labeling experiment.


 Please wait while we load your content...
Please wait while we load your content...