Two birds with one stone: one-pot simultaneous synthesis of 2,2,2-trifluoroethylphenanthridines and benzochromenones featuring the utilization of the byproduct of Togni's reagent†
Abstract
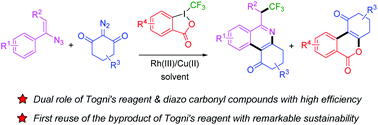
In this paper, a novel and efficient one-pot synthesis of 2,2,2-trifluoroethylphenanthridines (4) and benzochromenones (5) through three-component reactions of vinyl azides (1) with cyclic α-diazo carbonyl compounds (2) and Togni's reagent (3) is presented. In these cascade reactions, 3 acted not only as the source of a CF3 radical to react with 1 and 2 to afford 4, but also as the pool of o-iodobenzoic acid to couple with 2 to afford 5. To our knowledge, this is the first example in which 4 and 5 were obtained in a one-pot manner, and the byproduct of Togni's reagent, o-iodobenzoic acid, was trapped in situ by another substrate to give valuable products instead of being eliminated as a waste.


 Please wait while we load your content...
Please wait while we load your content...