Sustainable hydrogenation of aliphatic acyclic primary amides to primary amines with recyclable heterogeneous ruthenium–tungsten catalysts†
Abstract
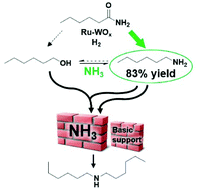
The hydrogenation of amides is a straightforward method to produce (possibly bio-based) amines. However current amide hydrogenation catalysts have only been validated in a rather limited range of toxic solvents and the hydrogenation of aliphatic (acyclic) primary amides has rarely been investigated. Here, we report the use of a new and relatively cheap ruthenium–tungsten bimetallic catalyst in the green and benign solvent cyclopentyl methyl ether (CPME). Besides the effect of the Lewis acid promotor, NH3 partial pressure is identified as the key parameter leading to high primary amine yields. In our model reaction with hexanamide, yields of up to 83% hexylamine could be achieved. Beside the NH3 partial pressure, we investigated the effect of the catalyst support, PGM-Lewis acid ratio, H2 pressure, temperature, solvent tolerance and product stability. Finally, the catalyst was characterized and proven to be very stable and highly suitable for the hydrogenation of a broad range of amides.


 Please wait while we load your content...
Please wait while we load your content...