Wealth from waste: blue mussels (Mylitus edulis) offer up a sustainable source of natural and synthetic nacre†
Abstract
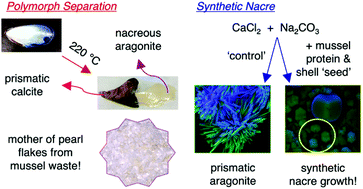
Blue mussel (Mylitus edulis) shells are composed of an outer prismatic calcite layer and an inner nacreous aragonite layer. They are a by-product of the seafood industry and a potential feedstock in the ocean- or marine-biorefinery. Heat treatment of these shells can yield four CaCO3 materials with differing ratios of aragonite and calcite, and separation of the aragonite and calcite. Infrared spectroscopy (IR) and powder X-ray diffraction (XRD) revealed that when shells are heated (120–220 °C, 48 h), the ratios of aragonite and calcite change. The organic matrix, made up of silk-fibroin like proteins, β-chitin and glycoproteins, was revealed by 1H MAS NMR spectroscopy and shows previously unreported resonances in mollusc shells at chemical shifts typical for amides and aromatic groups. Heat treatment of the shells caused a large decrease in organic matrix levels as shown by 1H MAS NMR spectroscopy and this reduction in matrix content leads to a simple way to separate the prismatic calcite layer from the nacreous layer, allowing easy isolation of natural platelets of nacre. The prismatic calcite layer still contains some organic matrix whereas only about 0.02 wt% matrix remains in the nacreous layer. Using mussel protein hydrolysate (another by-product of the seafood industry) as an additive, artificial nacre was synthesized using a CaCl2 and Na2CO3 mixing method. Good polymorph control was seen, and new platelets of nacre can be seen growing on the surface of a mussel shell that was used for seeding in the presence of mussel protein hydrolysate.


 Please wait while we load your content...
Please wait while we load your content...