Nickel-catalyzed intelligent reductive transformation of the aldehyde group using hydrogen†
Abstract
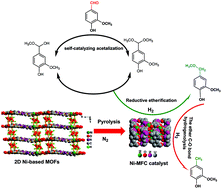
The selective transformation of the aldehyde group (–CHO) in multifunctional oxygenates is a key challenge in the development of sustainable biomass feedstock. Herein, a smart Ni-MFC catalyst was developed from a 2D Ni-based metal–organic framework (MOF), which efficiently promoted the transformation of –CHO in the presence of H2 to a methyl group (–CH3) via the reductive etherification and hydrogenolysis of the C–O ether bond in methanol. Moreover, the catalytic process could be controlled to directionally produce methyl ether (–CH2OR) using the reductive etherification protocol. For the catalytic reduction of vanillin, the Ni-MFC-700 catalyst guaranteed the full conversion of vanillin and 96.5% yield of the desired 2-methoxy-4-methylphenol (MMP), while the Ni-MFC-500 catalyst afforded about 82.7% yield of 4-(methoxymethyl)-2-methoxyphenol in methanol solvent. This is a novel and promising approach for the valorization of multifunctional oxygenates and biomass-derived platform compounds.


 Please wait while we load your content...
Please wait while we load your content...