Highly selective conversion of glyceric acid to 3-iodopropionic acid by hydriodic acid mediated hydrogenation†
Abstract
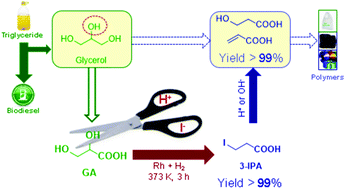
Glycerol, generated in abundance as the by-product in the process of biodiesel production and saponification, has seen attempts to convert it into value-added chemicals. However, due to the low selectivity of hydrogenolysis of the secondary hydroxyl group, valuable 1,3-substituted chemicals are difficult to obtain from glycerol by chemocatalysis. In this work, glyceric acid (GA), a renewable biomass from glycerol, was quantitatively converted to 3-iodopropionic acid (3-IPA) at 373 K in 3 h by hydroiodic acid mediated hydrogenation. As the reductant in this process, HI is oxidized to I2 and then regenerated in situ by metal catalysts and H2. The reaction pathway was proposed by intermediate identification and verified by a kinetics study and computational method. The catalytic system was shown to be stable and can be reused several times without loss in activity. As a 1,3-substituted chemical, 3-IPA is not only a potential monomer to form poly-3-hydroxypropionic acid, but also a good platform molecule to produce useful chemicals, e.g. 3-hydroxypropionic acid (3-HPA) and acrylic acid (AA), due to its highly reactive nature.


 Please wait while we load your content...
Please wait while we load your content...