Biocatalytic synthesis of panthenyl monoacyl esters in ionic liquids and deep eutectic solvents†
Abstract
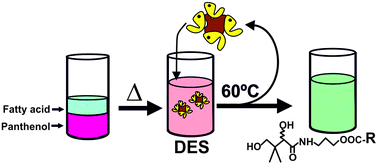
The enzymatic synthesis of six panthenyl monoacyl esters (PMEs) was carried out by the direct esterification of fatty acids (i.e. capric, lauric, myristic, palmitic, oleic and linoleic acids, respectively) with panthenol in different ionic liquids (ILs) based on cations with a long alkyl side-chain (e.g. 1-dodecyl-3-methylimidazolium tetrafluoroborate [C12mim][BF4], etc.). All the assayed ILs were seen to be suitable reaction media for Novozym 435-catalyzed synthesis of PMEs (i.e. up to 90% conversion and 100% selectivity), enabling easy recovery and the reuse of both biocatalyst and IL. Alternatively, mixtures of panthenol with free fatty acids were seen to act as deep eutectic solvents (DESs), that were excellent reaction media for the biocatalytic synthesis of PMEs (i.e. up to 83% conversion and 98% selectivity in the case of the panthenyl monolaurate), the enzymatic activity remaining unchanged for seven consecutive cycles of reuse. The enzymatic synthesis of PMEs by direct esterification using the DES approach can be considered as a clean and useful process for the sustainable industrial scaling up of panthenyl acyl ester production.


 Please wait while we load your content...
Please wait while we load your content...