Synthesis of functionalized 3-isochromanones via metal-free intramolecular alkoxylation-initiated cascade cyclization†
Abstract
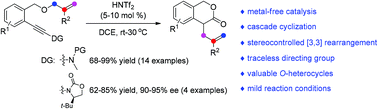
A metal-free intramolecular alkoxylation-initiated cascade cyclization of allyl ether-tethered ynamides has been developed. Various highly functionalized 3-isochromanones can be obtained in generally good to excellent yields under mild reaction conditions. Moreover, this asymmetric cyclization has also been realized via a stereocontrolled [3,3] rearrangement by employing a traceless chiral directing group. In addition, an unexpected [1,3] O-to-C rearrangement is observed in the case of the ynamide substrate bearing a phenyl-substituted alkene, which is distinctively different from the related gold catalysis.


 Please wait while we load your content...
Please wait while we load your content...