Boronic acid-promoted site-selective Fischer esterifications of sugar alcohols†
Abstract
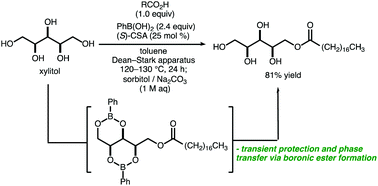
A protocol for selective monoesterification of glycerol and pentose alcohols with fatty acids is described, using phenylboronic acid as a phase-transfer reagent. Formation of arylboronic ester intermediates serves both to increase the solubility of the alcohol substrate in nonpolar organic solvent and to selectively protect diol groups, allowing for efficient and selective condensation with the aliphatic carboxylic acid. A phase-switching workup with basic aqueous sorbitol solution is used to cleave the boronic ester groups and to separate the boronic acid from the monoester product. The method provides an operationally simple means of access to a class of bio-derived products that have been broadly applied as food additives, components of cosmetic products and pharmaceutical formations, plasticizers, and non-ionic surfactants.


 Please wait while we load your content...
Please wait while we load your content...