A mild synthesis of substituted 1,8-naphthyridines†
Abstract
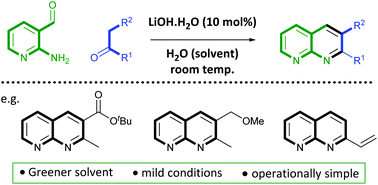
A greener method for the synthesis of substituted 1,8-naphthyridines has been developed, which is supported by reaction metric analysis. Using 2-aminonicotinaldehyde as a starting material with a variety of carbonyl reaction partners, the Friedländer reaction can be performed with high yield using water as the reaction solvent. Divergent reactivity was seen when using acrolein, and an alternative method was developed to give access to 2-vinyl-1,8-naphthyridine in high yield, and an assessment of addition reactions to 2-vinyl-1,8-naphthyridine was performed.


 Please wait while we load your content...
Please wait while we load your content...