Abstract
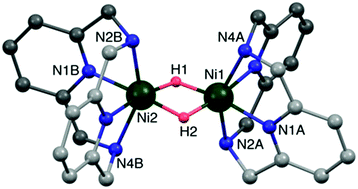
Transition metal-hydrides are highly useful in organic transformations of industrial importance yet synthesizing them or their precursor metal-borohydrides in high yield is cumbersome due to their high reactivity and sensitivity towards air and many common solvents. Reported is the development of a mechanochemical protocol for the solvent-free high-yield synthesis of nickel(II) borohydrides as precursors of nickel(II) hydrides. The Ni(II)-chelates (LHNi) were prepared by manually grinding Ni(II)-halides with a tetradentate macrocyclic N4 chelating ligand in the presence of air. The Ni(II)-chelates obtained were further ground with sodium borohydride to yield nickel(II)-borohydrides [LHNi(η2-BH4)]+, which in turn upon dissolution in solvents lost BH3 to yield homodinuclear nickel(II)-μ-hydrides [LHNi(μ-H)2NiL]2+. Representative reactions of the fully characterized nickel-hydride with alcohols, disulphides, benzyl bromide, trityl cation, air and SO2 yielding alkoxide, thiols, toluene, triphenylmethane, carbonate and sulfate, respectively, are presented. These results open a new avenue in handling sensitive borohydrides and provide a new route for high yield synthesis of hydrides and their precursors.


 Please wait while we load your content...
Please wait while we load your content...