Utilization of bio-based glycolaldehyde aqueous solution in organic synthesis: application to the synthesis of 2,3-dihydrofurans†
Abstract
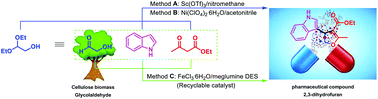
Glycolaldehyde is a biomass-derived chemical compound available from cellulose or glucose. Until now, little attention has been devoted to its use towards value-added chemicals. To explore novel transformations of glycolaldehyde, in this work, a three-component reaction of glycolaldehyde, indole and a 1,3-dicarbonyl compound was developed to synthesize a class of 3-(indol-3-yl)-2,3-dihydrofurans. Using glycolaldehyde diethyl acetal as a glycolaldehyde source, the reaction can be performed in organic solvents, and two catalytic systems were proved to be effective: (a) Sc(OTf)3/nitromethane and (b) Ni(ClO4)2·6H2O/acetonitrile. However, these conditions applied to the direct use of the bio-based glycolaldehyde aqueous solution did not provide the dihydrofurans efficiently. To enable the use of an aqueous solution of glycolaldehyde, a hitherto unreported deep eutectic solvent (DES) was developed by using FeCl3·6H2O and meglumine (N-methylglucamine) as precursors. The FeCl3·6H2O/meglumine DES was characterized by FTIR, TGA and DSC, and the obtained results demonstrated unambiguously the formation of a DES. This DES was found to be an efficient and water-compatible promoting medium for the abovementioned three-component reaction. A variety of 3-(indol-3-yl)-2,3-dihydrofurans were synthesized in good yields. The FeCl3·6H2O/meglumine DES system can also be recycled without significant loss of activity.


 Please wait while we load your content...
Please wait while we load your content...