Access to green primary explosives via constructing coordination polymers based on bis-tetrazole oxide and non-lead metals†
Abstract
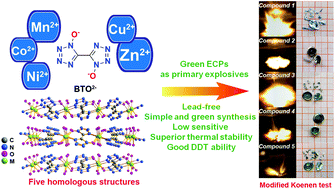
An environmentally friendly method to prepare new-generation green primary explosives with excellent performances has been developed on the basis of a coordination chemistry strategy. In the present work, constructing energetic coordination polymers (ECPs) based on tetrazole oxide 1H,1′H-[5,5′-bitetrazole]-1,1′-diol (H2BTO) with non-heavy metal cations Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) led to five novel green primary explosives without metal ion and organic pollution. Systematic research is conducted to prove these green ECPs as a novel class of lead-free primary explosives. For example, a thermal stability experiment shows that their thermal decomposition temperatures are all beyond 200 °C, while the thermal-resistance experiment demonstrates that compounds 1 and 5 can maintain long-term stability under 100 °C. And the results based on sensitivity tests and detonation parameter simulation reveal that all these compounds possess excellent detonation and safety properties, far outperforming classical primary explosives such as lead azide. Moreover, a modified Koenen test has been conducted to characterize the explosion capacity of these ECPs and by this method compounds 1, 2, 3, and 5 were demonstrated with fast deflagration-to-detonation transition ability.


 Please wait while we load your content...
Please wait while we load your content...