Ionic liquid catalysed aerobic oxidative amidation and thioamidation of benzylic amines under neat conditions†
Abstract
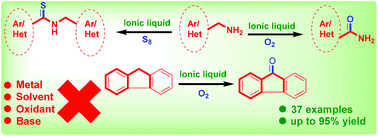
Tetrabutylammonium hydroxide (TBAOH) was discovered as a highly efficient and green catalyst for aerobic oxidation of the α-methylene carbon of primary amines as well as benzylic groups into the corresponding amides and ketones under neat conditions. We described herein, ionic liquid TBAOH catalysed aerobic oxidation of benzyl amines to benzamides and with elemental sulfur; the corresponding benzylbenzothioamides were obtained under metal-free, oxidant-free and base-free conditions. Applicability at the gram scale for the synthesis of the desired amides/ketones is also demonstrated with the present protocol.


 Please wait while we load your content...
Please wait while we load your content...