Eco-friendly decarboxylative cyclization in water: practical access to the anti-malarial 4-quinolones†
Abstract
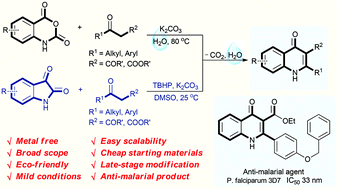
An environmentally benign decarboxylative cyclization in water has been developed to synthesize 4-quinolones from readily available isatoic anhydrides and 1,3-dicarbonyl compounds. Isatins are also compatible for the reaction to generate 4-quinolones in the presence of TBHP in DMSO. This protocol provides excellent yields under mild conditions for a broad scope of 4-quinolones, and has good functional group tolerance. Only un-harmful carbon dioxide and water are released in this procedure. Moreover, the newly synthesized products have also been selected for anti-malarial examination against the chloroquine drug-sensitive Plasmodium falciparum 3D7 strain. 3u is found to display excellent anti-malarial activity with an IC50 value of 33 nM.


 Please wait while we load your content...
Please wait while we load your content...