Comparative study on the interaction of oxyresveratrol and piceatannol with trypsin and lysozyme: binding ability, activity and stability†
Abstract
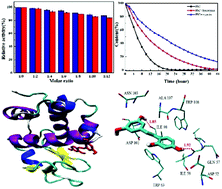
Natural polyphenols showing a variety of beneficial effects will interact with multiple proteases after administration. The interactions of oxyresveratrol and piceatannol with trypsin and lysozyme were investigated using fluorescence spectroscopy, UV-vis absorption spectroscopy, circular dichroism spectroscopy, differential scanning calorimetry and molecular docking. Fluorescence quenching results and UV-vis absorption difference spectra revealed that the quenching process was a static mode initiated by ground-state complex formation. The different binding ability of oxyresveratrol and piceatannol with trypsin and lysozyme was discussed based on their different molecular structures. Moreover, the major driving force for the binding process was elucidated as hydrogen bonding and van der Waals forces by the negative enthalpy and entropy changes. Synchronous fluorescence, three-dimensional fluorescence and circular dichroism spectral analysis suggested that the binding of oxyresveratrol and piceatannol to trypsin and lysozyme induced some microenvironmental and conformational changes of the two enzymes. The thermal stability of the enzymes in the presence of polyphenols was studied based on the change in melting temperature by differential scanning calorimetry. The above experimental results were validated by the protein–ligand docking studies which showed the location of the two ligands in the enzymes and the surrounding amino acid residues. Furthermore, enzyme activity assays indicated that the enzymatic activity of trypsin and lysozyme was inhibited by oxyresveratrol and piceatannol. The effect of trypsin and lysozyme on the antioxidant activity and stability of oxyresveratrol and piceatannol was also investigated. In conclusion, the comparative study on the interaction of oxyresveratrol and piceatannol with trypsin and lysozyme showed that the positions of hydroxyl groups of the polyphenols had an important influence on their interaction with enzymes and their antioxidant activity and stability as well as the enzyme activities. The obtained results are expected to provide a theoretical basis for the application of polyphenols in functional foods and pharmaceuticals.

 Please wait while we load your content...
Please wait while we load your content...