Formation of fibrils derived from whey protein isolate: structural characteristics and protease resistance†
Abstract
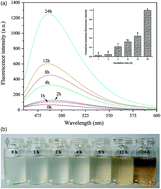
The formation of lactoglobulin (β-lg) fibrils from its native globular form has been investigated hitherto with pure lactoglobulin conditions. For commercially feasible production of β-lg fibrils for food and therapeutic applications, the effect of impurities (albeit at very small fractions) found in industrial grade lactoglobulin proteins will need to be considered. In this work, food grade whey protein isolate (WPI), with its associated trace globulin and other impurities, was used to study the β-lg fibril formation process. SDS-PAGE analysis supported by Thioflavin T measurement showed that the WPI fibril formation accompanied by hydrolysis limited the formation reaction rate; further analysis indicated that main impurities especially for α-lactalbumin may affect the intermolecular contacts among β-lg. The increase of β-sheet and α-helix conformation analyzed by circular dichroism revealed that the β strand axis with an α-helix stabilized the linear structure of fibrils. The WPI fibrils exhibited a significant variation in lengths and differences in morphology when compared to some other protein fibrils reported in the literature. Two-stage pepsin followed by pancreatin digestion analysis revealed that the WPI fibrils produced will have the potential as a medium for active compound delivery to the intestinal environment.

 Please wait while we load your content...
Please wait while we load your content...