Photocatalytic reduction of chlorite in water using bismuth vanadate (BiVO4): effect of irradiance conditions and presence of oxalate on the reactivity and by-product selectivity†
Abstract
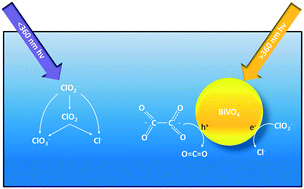
Chlorite (ClO2−) is a disinfection byproduct formed during drinking water treatment when source waters with a high oxidant demand are disinfected with chlorine dioxide. This study investigated the reduction of ClO2− using a visible light (vis) active photocatalyst, bismuth vanadate (BiVO4). The effect of direct photolysis, the presence of oxalate, light source, and fluence on the reaction rate and by-product selectivity was evaluated. A commercial titanium dioxide (TiO2) was used as a UV-benchmark comparison. Photolysis was an effective reductant of ClO2−, but it produced the undesirable byproduct chlorate (ClO3−) and required ultraviolet light (UV) less than 360 nm. BiVO4 and TiO2 reduced ClO2− using irradiation greater than 360 nm, but ClO3− was also produced. For BiVO4, the addition of oxalate as a hole scavenger and dissolved organic carbon (DOC) analog increased the reaction rate and eliminated ClO3− formation, but it decreased the reaction rate for TiO2 and ClO3− was still detected. In situ vibrational spectroscopy was used to link the reduced rate of TiO2 to the chemisorption behavior between oxalate and TiO2. Analyzing the reaction rates as a function of photon fluence instead of time showed BiVO4 was more efficient at using vis than UV. A higher required energy dose for the 2-log removal of ClO2− was observed for BiVO4 (185 J cm−2) compared to TiO2 (5.11 J cm−2), indicating the poor ability of BiVO4 to separate and use photogenerated charge carriers. The addition of silver to enhance charge separation improved the efficiency and reduced the required dose (45.5 J cm−2). From an operational standpoint, this study strengthens the argument that all photocatalysis treatment studies should report results as a function of fluence as well as time. Though BiVO4 used more energy than TiO2 to achieve the same removal, its ability to avoid ClO3− is attractive. Further, vis sources are more cost and energy efficient with longer lifetimes compared to UV sources, and advanced materials engineering can improve reaction efficiencies, allowing vis photocatalysts like BiVO4 to be an attractive option for treatment of ClO2− and other critical oxidized contaminants.


 Please wait while we load your content...
Please wait while we load your content...