Insights into the facet-dependent adsorption of phenylarsonic acid on hematite nanocrystals†
Abstract
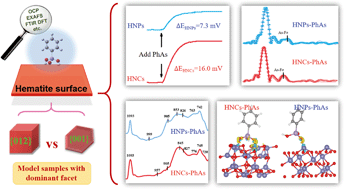
Organic arsenics have been frequently detected in natural environments and regarded as a group of emerging contaminants. Elucidating the migration of organic arsenics requires a deep understanding of intrinsic adsorption mechanisms of organic arsenics on iron-bearing minerals, which are seldom investigated. In this study, we investigated the facet-dependent adsorption behavior of phenylarsonic acid (PhAs) on hematite nanocrystals with open circuit potential (OCP) analysis, batch adsorption experiments, adsorption isotherm curves, synchrotron-based As K-edge extended X-ray absorption fine structure (EXAFS) spectroscopy, attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy, and density functional theory (DFT) calculations. We found that organic arsenics adsorbed on two facets (e.g. {001} and {012}) of hematite through the same non-protonated inner-sphere coordination but in different molecular configurations. Surface complexation models were set up to analyze the preferred coordination geometries dependent on the facets, and the {012} facet was found to favor the PhAs adsorption more than the {001} facet. These findings clarified the dependence of organic arsenic adsorption on the hematite facets, and shed light on the environmental effects of hematite on the migration of PhAs.


 Please wait while we load your content...
Please wait while we load your content...