Facet-selective adsorption of Fe(ii) on hematite visualized by nanoscale secondary ion mass spectrometry†
Abstract
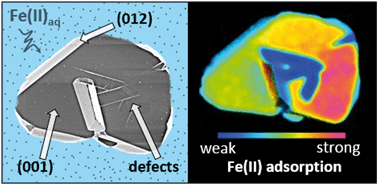
Facet-specific reactivity of metal oxide particles is a well-known but at times difficult to probe phenomenon. Furthermore, in semiconductor metal oxides where crystal facets enclosing particles are electrically connected, separating them to enable detailed characterization defeats the purpose; the study of intact individual crystallites is necessary. Here we develop a mass-sensitive imaging approach to do so, and demonstrate its potential by unveiling the preferential binding of Fe(II) to various surfaces of the Fe(III) oxide hematite. Using isotopic tracers to follow iron provenance, 56Fe-hematite microplatelets with various enclosing facets are reacted with aqueous 57Fe(II) at circumneutral pH. The resulting distribution of 57Fe across the hematite surfaces is directly visualized and quantified using nanoscale secondary ion mass spectrometry (NanoSIMS). The results unambiguously show Fe(II) sorption is highly selective for the basal (001) surface, while edge surfaces such as (012) and (110) are enriched to a lesser extent (up to 10× lower). Crystal intergrowth defects exposing poorly-ordered, nanoscale surface structures show the least enrichment. These results resolving Fe(II)–Fe(III) reaction fronts across multi-facetted crystals provide a clear correlation between uptake and particle surface structure. The illustrated approach to understanding facet-specific ion uptake is also likely generalizable to other interfacial processes such as electron transfer and heterogeneous catalysis, across a broad range of particle and thin-film based systems.


 Please wait while we load your content...
Please wait while we load your content...