Synergistic catalysis of N vacancies and ∼5 nm Au nanoparticles promoted the highly sensitive electrochemical determination of lead(ii) using an Au/N-deficient-C3N4 nanocomposite†
Abstract
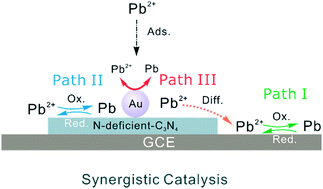
Most nanomaterials with good adsorption properties are restricted from being applied in electrochemical detection due to the lack of active sites and poor electrocatalytic reactivity. Here, we found a synergistic catalysis effect of N vacancies and ∼5 nm Au nanoparticles in Au/N-deficient-C3N4 for highly sensitive electrochemical detection of Pb(II) with anti-interference via a defect-engineering strategy. N vacancy defects were introduced into g-C3N4 to prepare N-deficient-C3N4, which showed a significantly enhanced electrochemical sensitivity toward Pb(II) (689.0 μA μM−1 cm−2). The sensitivity of Au/N-deficient-C3N4 dramatically rose to 1223.0 μA μM−1 cm−2 because of the synergistic catalysis effect, which was approximately 4 times as large as that of pure g-C3N4. The N vacancies in g-C3N4 could greatly improve the selective adsorption of Pb(II), and ∼5 nm Au nanoparticles enhanced the catalysis of materials. High concentration of other common ions (Hg(II), Cu(II), or Cd(II)) did not interfere in the electrochemical detection of Pb(II). A strong chemical interaction between Pb(II) and Au/N-deficient-C3N4 was confirmed by X-ray photoelectron spectroscopy results, which was possibly responsible for the anti-inference properties. This work provides a new method to make semiconductors with intrinsically poor electrochemical activity potential materials in the electrochemical analysis field by defect-engineering and synergistic catalysis.


 Please wait while we load your content...
Please wait while we load your content...