Theoretical investigation of the mechanism, kinetics and subsequent degradation products of the NO3 radical initiated oxidation of 4-hydroxy-3-hexanone†
Abstract
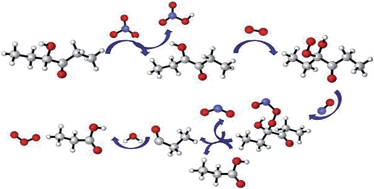
The oxidation mechanism of 4-hydroxy-3-hexanone (CH3CH2C(O)CH(OH)CH2CH3) initiated by NO3 radicals in the nighttime is investigated systematically by applying quantum theoretical methods. According to thermodynamic research, the process of H-abstraction on the –CH– group adjacent to the hydroxyl group is the most dominant pathway with the lowest activation energy. The analysis of Mulliken charge charts and molecular electrostatic potential maps illustrate that C–H bonds are the active sites of the reaction, and the calculated C–H bond dissociation energy of the CH3CH2C(O)CH(OH)CH2CH3 molecule further confirms that α-CH is the most easily activated. Individual rate constants for five H-abstraction pathways are calculated by canonical variational theory coupled with small curvature tunneling method over the temperature range of 260–330 K, and the branching ratios are also evaluated. A total rate constant of 1.18 × 10−15 cm3 per molecule per s is obtained at 298 K, which is in good agreement with the reported experimental value. A negative temperature dependence is observed in the titular reaction. The subsequent degradation processes of the advantageous product alkyl radical (CH3CH2C˙(OH)COCH2CH3) are carried out in a NO-rich environment, and propionic acid, NO2 and ozone are obtained as the major final products. The nighttime atmospheric lifetime of 4-hydroxy-3-hexanone is estimated to be around 19 days, indicating that it has impact at night. The titular reaction rate constants are fitted to a three-parameter Arrhenius formula.


 Please wait while we load your content...
Please wait while we load your content...