Electrochemomechanics of lithium dendrite growth†
Abstract
A comprehensive roadmap describing the current density- and size-dependent dendrite growth mechanisms is presented. Based on a thermodynamically consistent theory, the combined effects of chemical diffusion, electrodeposition, and elastic and plastic deformation kinetics are analyzed to rationalize their contributions to experimentally observable morphologies. A critical current density, î* = z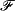 iliml(ΔGΩκi), in the tσ < t < tSand range, results in plastic flow at the tips, dendrite bifurcation, and bent and kinked morphologies. Three dendrite growth mechanisms are observed: (1) electrochemical shielding, where there is practically no electrodeposition/electrodissolution; (2) stress-induced electrodissolution and electrodeposition on those interfaces directly facing each other, generating a self-sustained overpotential that pushes the dendrites towards the counter electrode; and (3) local, lateral plastic extrusion in those side branches experiencing non-hydrostatic stresses. Six regimes of lithium electrodeposit growth are identified: (i) thermodynamic suppression regime, (ii) incubation regime, (iii) base-controlled regime, (iv) tip-controlled regime, (v) mixed regime, and (vi) Sand's regime.
iliml(ΔGΩκi), in the tσ < t < tSand range, results in plastic flow at the tips, dendrite bifurcation, and bent and kinked morphologies. Three dendrite growth mechanisms are observed: (1) electrochemical shielding, where there is practically no electrodeposition/electrodissolution; (2) stress-induced electrodissolution and electrodeposition on those interfaces directly facing each other, generating a self-sustained overpotential that pushes the dendrites towards the counter electrode; and (3) local, lateral plastic extrusion in those side branches experiencing non-hydrostatic stresses. Six regimes of lithium electrodeposit growth are identified: (i) thermodynamic suppression regime, (ii) incubation regime, (iii) base-controlled regime, (iv) tip-controlled regime, (v) mixed regime, and (vi) Sand's regime.



 Please wait while we load your content...
Please wait while we load your content...