Bisalt ether electrolytes: a pathway towards lithium metal batteries with Ni-rich cathodes†
Abstract
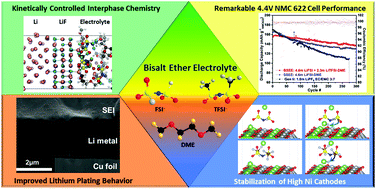
The electrochemical performance and mechanistic effects of incorporating two salts in an ether electrolyte in Li–metal cells were investigated experimentally and via molecular scale modeling. Improvements in efficiency and cycling stability over baseline electrolytes in lithium (Li) versus copper (Cu) cells were directly correlated to the initial Li-nucleation process, as observed via cryogenic-focused ion beam (cryo-FIB) prepared cross sections, which revealed that Li films deposited with the bisalt electrolyte were significantly thinner and denser than those from the baseline. This behavior was further traced back to the initial nucleation process via cryogenic transmission electron microscopy (cryo-TEM), which shows stark differences in the morphology and conformality of deposited Li as a function of electrolyte chemistry. X-ray photoelectron spectroscopy (XPS) indicated that the solid electrolyte interphase (SEI) formed from the bisalt electrolyte primarily consists of larger anion fragments, suggesting that the anion chemistry strongly influences the extent of reduction and resulting surface chemistry. Ab initio DFT-based and force field-based molecular dynamics calculations revealed the complex interplay (positioning, orientation, reactivity, kinetics) between the FSI− and TFSI− anions in the double layer at both electrode–electrolyte interfaces and the ramifications for stabilizing these interfaces. As a result of the unique interphasial chemistry brought by this bisalt system, this electrolyte supports an unprecedented (for an ether-based electrolyte) capacity retention (>88%) after 300 cycles (∼2 months of cycling) in a 4.4 V NMC622-Li cell, and a >30% improvement in capacity retention over baseline after 50 cycles in 4.4 V NMC622-Cu “anode-free” cells. Altogether, these results provide new insight into how the bisalt effect can be leveraged for regulating the timescale, chemistry, and extent of interfacial reactions. When balanced properly, this promotes efficient plating/deplating of Li, and potentially supports widespread implementation of high nickel content NMC cell configurations with limited or no excess lithium.


 Please wait while we load your content...
Please wait while we load your content...