Enhancing the activity of oxygen-evolution and chlorine-evolution electrocatalysts by atomic layer deposition of TiO2†
Abstract
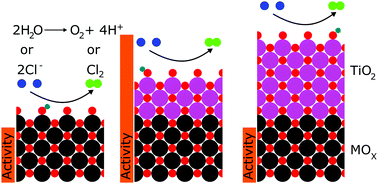
We report that TiO2 coatings formed via atomic layer deposition (ALD) may tune the activity of IrO2, RuO2, and FTO for the oxygen-evolution and chlorine-evolution reactions (OER and CER). Electrocatalysts exposed to ∼3–30 ALD cycles of TiO2 exhibited overpotentials at 10 mA cm−2 of geometric current density that were several hundred millivolts lower than uncoated catalysts, with correspondingly higher specific activities. For example, the deposition of TiO2 onto IrO2 yielded a 9-fold increase in the OER-specific activity in 1.0 M H2SO4 (0.1 to 0.9 mA cmECSA−2 at 350 mV overpotential). The oxidation state of titanium and the potential of zero charge were also a function of the number of ALD cycles, indicating a correlation between oxidation state, potential of zero charge, and activity of the tuned electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...