Cerium–carbon dative interactions supported by carbodiphosphorane†
Abstract
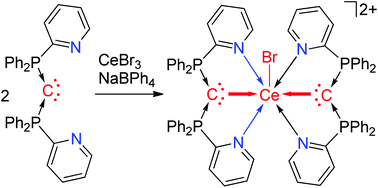
A set of complexes containing dative interactions between a rare-earth metal and carbon are reported. Complex 2, Br3Ce(CDP)(THF), with a Ce←C bond was synthesized by the reaction of CeBr3 with a carbon(0) ligand, carbodiphosphorane (CDP). More significantly, a trivalent cerium complex 3, [BrCe(CDP)2](BPh4)2, with two σ dative interactions C→Ce←C was also isolated, which represents an unusual example of two dative interactions formed with the same atom in a molecule. Furthermore, π donation by the second lone-pair electrons of the CDP ligand is rather weak. Single-crystal X-ray diffraction shows that the Ce–C bond lengths in these complexes are comparable with those in cerium(III)–carbene species. Density functional theory calculations support the dative interaction formation in these complexes and the strength of σ-donation in 3 is stronger than that in 2.


 Please wait while we load your content...
Please wait while we load your content...