Cyclotriveratrylene-tethered trinuclear palladium(ii)–NHC complexes; reversal of site selectivity in Suzuki–Miyaura reactions†‡
Abstract
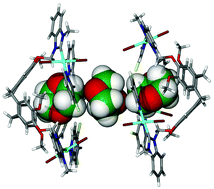
The trinuclear complexes [{PdI2(pyCl)}3(L1)] C1 and [{PdI2(pyCl)}3(L2)] C2, where pyCl = 3-chloropyridine, L1 = methyl(cyclotriguaiacylenyl)methylbenzimidazol-2-ylidene and L2 = benzyl(cyclotriguaiacylenyl)methylbenzimidazol-2-ylidene, each feature three palladium N-heterocyclic carbene (NHC) centres tethered onto a host-type cyclotriguaiacylene scaffold. Crystal structures of different solvates of complex C1 reveal different host–guest motifs including intra-cavity binding of dioxane guests concomitant with intramolecular halogen bonding interactions of C1. Mononuclear NHC analogues of C1 and C2, namely [PdI2(pyCl)(L3)] C3 and [PdI2(pyCl)(L4)] C4, where L3 = (3-chloropyridyl)-1-(2-methoxyphenyoxy)methyl-3-methylbenzimidazol-2-ylidene and L4 = (3-chloropyridyl)-1-(2-methoxyphenyoxy)methyl-3-benzylbenzimidazol-2-ylidene, were also synthesised and their crystal structures determined. Complexes C1–C4 are competent catalysts for Suzuki Miyaura cross-coupling, and interestingly exhibit a switch in the normal regioselectivity observed for reactions of 2,4-dibromopyridine with aryl boronic acids, usually C2-selective, yielding C4-arylated product preferentially over C2-arylated product.


 Please wait while we load your content...
Please wait while we load your content...