Electron transfer in complexes of BII cations with organic π-acceptors: a combined experimental and quantum-chemical study†
Abstract
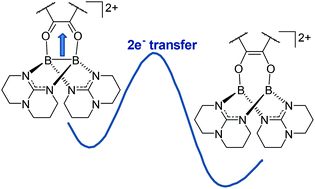
Due to their combined Lewis acidity and electron-donor capability, BII cations exhibit an interesting reactivity, which is almost unexplored so far. In this work, we compare the reduction in a dicationic diborane of a series of vicinal diones with different redox potentials, namely 3,5-di-tert-butylbenzoquinone, 3,4,5,6-tetrachlorobenzoquinone, 1,2-naphthalene-dione, 9,10-phenanthrene-dione, 2,2′-dichlorobenzil, benzil and 1,2-acenaphthylene-dione. The experimental work is complemented by quantum-chemical calculations, illuminating the electron-transfer step in the reactions.


 Please wait while we load your content...
Please wait while we load your content...