Unusual C–O bond cleavage of aromatic ethers in ruthenium complexes bearing a 2-alkoxypyridyl fragment†
Abstract
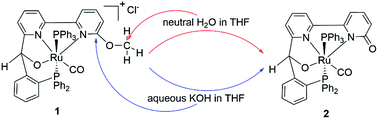
Two tetradentate (NNOP) ruthenium products [{RO-C5H3N-C5H3N-CH(O)-C6H4-PPh2}Ru(CO)(PPh3)]Cl (1: R = Me; 3: R = Ph) were isolated by the reactions of the corresponding ligands with RuHCl(PPh3)3(CO). Complex 1 could also be transformed into 2 when heated in THF, through C–OMe bond cleavage. The mechanism of the unusual C–O cleavage in complex 1 was investigated, and the results indicate that it is an SN2 mechanism through the attack of trace amounts of neutral H2O. In contrast, in the presence of KOH, the reaction switches to an SNAr chemistry. The bidentate ruthenium isomers [{CH3O-C5H3N-C5H4N}RuH(CO)(PPh3)2]Cl (4a and 4b) could undergo similar C–O cleavage, affording product {O-C5H3N-C5H4N}RuH(CO)(PPh3)2 (5), which reacted with HCl to generate complex [{HO-C5H3N-C5H4N}RuH(CO)(PPh3)2]Cl (6). These complexes were tested as catalysts for β-alkylation of secondary alcohols with primary alcohols, and a series of β-alkylated 1-phenylethanol derivatives were isolated in high yields.


 Please wait while we load your content...
Please wait while we load your content...