Water soluble, optically active monofunctional Pd(ii) and Pt(ii) compounds: promising adhesive and antimigratory effects on human prostate PC-3 cancer cells†
Abstract
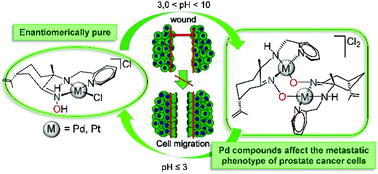
New water soluble, enantiopure palladium and platinum compounds RN-[M{(1S,4R)-κNOH,κ2NH(2-pic)}Cl]Cl and SN-[M{(1R,4S)-κNOH,κ2NH(2-pic)}Cl]Cl (2-pic = 2-picolyl, M = Pd 1 and 1′, Pt 2 and 2′, respectively), and heterometallic Pd/Ti [(η5-C5H5)2Ti{(1S,4R)-κON,κ2NH(2-pic)}(PdCl)]Cl (3) have been synthesized. These novel compounds were fully characterized by NMR spectroscopy and CHN elemental analysis and 1, 1′, 2 and 2′ were further evaluated by polarimetry, ultra-violet and circular dichroism spectroscopy. The aqueous stability of novel compounds was studied by NMR spectroscopy under physiological conditions and the new species detected under such conditions have been characterized by NMR techniques and HR-ESI-MS (High-Resolution Electrospray Ionization Mass Spectrometry). Compound–DNA interactions have been investigated for the palladium and platinum compounds by equilibrium dialysis, Fluorescence Resonance Energy Transfer (FRET) DNA melting assays and viscometric titrations, revealing a better binding affinity and ability to affect duplex DNA of the palladium compounds. Metal derivatives have been tested in vitro against three cancer (prostate PC-3, cervical HeLa and breast MCF-7) and one non-tumorigenic (human prostate RWPE-1) cell lines. The highest anticancer activities were shown by palladium compounds 1 and 1′ in all cancer lines, although their toxicity was lower than that found for cisplatin. Most importantly, the effect of the compounds on the cell adhesion and migration of the androgen-independent prostate cancer PC-3 cells has been assessed, and the efficacy of Pd enantiomers to affect the invasive phenotype of PC-3 cells has been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...