Synthesis and reactivity of boryl substituted silaimines†
Abstract
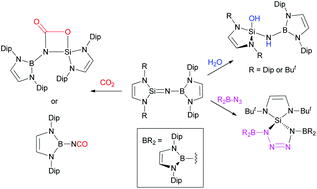
Reactions of two N-heterocyclic silylenes with a boron azide have given the first N-boryl substituted silaimines, (HCNR)2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N{B(DipNCH)2} (R = Dip or But, Dip = 2,6-diisopropylphenyl) which are either monomeric or dimeric in the solid state. The compounds shows divergent reactivity with CO2, in that the bulkier silaimine undergoes a [2+2] cycloaddition with CO2 to form (HCNDip)2Si{OC(
N{B(DipNCH)2} (R = Dip or But, Dip = 2,6-diisopropylphenyl) which are either monomeric or dimeric in the solid state. The compounds shows divergent reactivity with CO2, in that the bulkier silaimine undergoes a [2+2] cycloaddition with CO2 to form (HCNDip)2Si{OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)N[B(DipNCH)2]} under mild conditions, while the smaller silaimine undergoes a bond metathesis reaction with CO2, affording the boron isocyanate, (HCNDip)2B(NCO), and a known oxo-bridged silicon(IV) system. Both silaimines add H2O across their Si
O)N[B(DipNCH)2]} under mild conditions, while the smaller silaimine undergoes a bond metathesis reaction with CO2, affording the boron isocyanate, (HCNDip)2B(NCO), and a known oxo-bridged silicon(IV) system. Both silaimines add H2O across their Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds to give the boryl-aminosilanols, (HCNR)2Si(OH)–(H)N{B(DipNCH)2}. The larger silaimine does not react with the bulky boron azide (HCNDip)2BN3, whereas the smaller system undergoes a [3+2] cycloaddition reaction with the azide to give spirocyclic borasilatetrazoline, (HCNBut)2Si{[(HCNDip)2B]NN}2. Overall, the reactivity of the prepared N-boryl substituted silaimines is similar to that reported for other silaimines. However, the steric differences between the two Si
N bonds to give the boryl-aminosilanols, (HCNR)2Si(OH)–(H)N{B(DipNCH)2}. The larger silaimine does not react with the bulky boron azide (HCNDip)2BN3, whereas the smaller system undergoes a [3+2] cycloaddition reaction with the azide to give spirocyclic borasilatetrazoline, (HCNBut)2Si{[(HCNDip)2B]NN}2. Overall, the reactivity of the prepared N-boryl substituted silaimines is similar to that reported for other silaimines. However, the steric differences between the two Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonded compounds plays a definitive role in the outcomes of their reactions with the small molecule substrates studied.
N bonded compounds plays a definitive role in the outcomes of their reactions with the small molecule substrates studied.


 Please wait while we load your content...
Please wait while we load your content...