Controllable syntheses and magnetic properties of novel homoleptic triple-decker lanthanide complexes†
Abstract
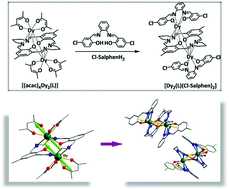
Four novel homoleptic triple-decker lanthanide complexes, [Ln2(L)(Cl-salphen)2]·0.5ClCH2CH2Cl (Ln3+ = Gd3+ (1), Ho3+ (2), Tb3+ (3), and Dy3+ (4)), have been designed and synthesized based on a Schiff base ligand N,N′-bis(5-chlorosalicylidene)-o-phenylenediamine (Cl-salphenH2) and the building blocks [(acac)4Ln2(L)]; these complexes were derived from a closed-macrocyclic ligand (H2L) obtained by the [2 + 2] template condensation of 2,6-diformyl-4-methylphenol and 1,3-diaminopropane in the presence of lanthanide acetylacetonates. Single-crystal X-ray analyses show that central Ln3+ ions adopt distorted square antiprism conformations with D4d symmetry. Theoretical analysis and magnetic measurements reveal that Dy complex 4 behaves as a typical SMM with the characteristics of slow magnetic relaxation behavior and intermetallic ferromagnetic interaction.


 Please wait while we load your content...
Please wait while we load your content...