Reversible uptake of sulfur-containing gases by single crystals of a Cr8 metallacrown†
Abstract
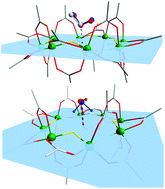
The exposure of green crystals of the [CrF(O2CtBu)2]8Cr8 metallacrown to SO2 and H2S gases results in the binding of the gas molecules in the internal molecular cavity, despite the absence of any pores or channels in the structure. Single crystal X-ray diffraction studies show that the gas molecules bind weakly to the bridging fluoride ligands. The desorption process was followed by TGA, DSC and in situ variable temperature single crystal X-ray diffraction, obtaining the gas binding energies for the gas guest molecules. These results are supported by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...