A readily accessible and modular carbohydrate-derived thioether/selenoether-phosphite ligand library for Pd-catalyzed asymmetric allylic substitutions†
Abstract
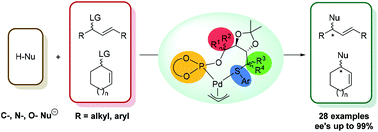
A large library of thioether/selenoether-phosphite ligands have been tested in the Pd-catalyzed asymmetric allylic substitution reaction. The presented ligands are derived from cheap and available carbohydrates and they are air-stable solids and easy to handle. Their highly modular nature has made it possible to achieve excellent enantioselectivities in the substitution of a range of hindered and unhindered substrates (ees up to 99% and 91%, respectively). In addition, twelve C-, N- and O-nucleophiles can be efficiently introduced, independently of their nature. Among the whole library, ligands that contain an additional chiral centre in the alkyl backbone chain next to the phosphite group and an enantiopure biaryl phosphite group provided the best enantioselectivities. In general, there is a cooperative effect between these two chiral elements, and therefore, a matched combination between them is necessary to achieve the highest enantioselectivities. However, in the case of cyclic substrates, this cooperative effect is less pronounced and advantageously, both enantiomers of the product can be obtained by setting up the desired configuration of the biaryl phosphite group. Studies of the key Pd–π-allyl intermediates allowed us to better understand the enantioselectivities obtained experimentally.


 Please wait while we load your content...
Please wait while we load your content...