In situ formed oxy/hydroxide antennas accelerating the water dissociation kinetics on a Co@N-doped carbon core–shell assembly for hydrogen production in alkaline solution†
Abstract
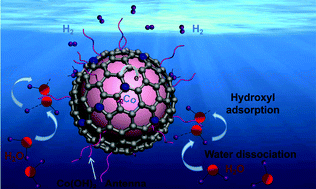
The hydrogen evolution reaction (HER) in alkaline electrolytes is restricted severely by sluggish water dissociation in the Volmer step. Here, we embedded Co crystals into a N-doped carbon (Co@NC) on nickel foam (NF) framework and electrochemically oxidized them in situ to construct a CoOxHy [a mixture of Co(OH)2 and CoOOH] antenna-modified Co@NC core–shell assembly (NF/Co@NC/CoOxHy). The CoOxHy antennas significantly decreased the activation energy of water dissociation to protons (the Volmer step), and hydrogen production over the Co core activated NC shell may follow a multi-carbon catalytic mechanism activated by Co and N atoms. As a result, the NF/Co@NC/CoOxHy configuration exhibits a 40 mV onset potential, a low HER overpotential of 51 mV at 10 mA cm−2, and a current density still reaching around 20 mA cm−2 after 55 h of stability testing in alkaline electrolyte. Our material functionalization strategy may open up a new approach for developing water-alkali electrolysers for use in efficient renewable energy conversion.


 Please wait while we load your content...
Please wait while we load your content...