Modular triazine-based carborane-containing carboxylic acids – synthesis and characterisation of potential boron neutron capture therapy agents made of readily accessible building blocks†
Abstract
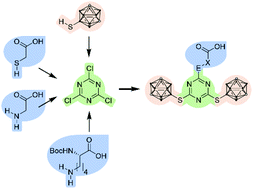
Based on a modular combination of s-triazine, the well-known 9-mercapto-1,7-dicarba-closo-dodecaborane(12) and commercially available carboxylic acids, namely thioglycolic acid, glycine, and Nα-Boc-L-lysine, several carboxylic acid derivatives were synthesised and fully characterised. The thioglycolic acid derivative was introduced into a peptide hormone by solid phase peptide synthesis. High activity and selective internalisation into peptide receptor-expressing cells was observed. With a very high boron content of twenty boron atoms, these derivatives are interesting as selective Boron Neutron Capture Therapy (BNCT) agents.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...