Photolysis of Tp′Rh(CNneopentyl)(PhNCNneopentyl) in the presence of ketones and esters: kinetic and thermodynamic selectivity for activation of different aliphatic C–H bonds†‡
Abstract
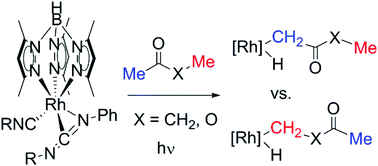
The active fragment [Tp′Rh(CNneopentyl)], generated from the precursor Tp′Rh(CNneopentyl)(PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNneopentyl), underwent oxidative addition of substituted ketones and esters resulting in Tp′Rh(CNneopentyl)(R)(H) complexes (Tp′ = tris-(3,5-dimethylpyrazolyl)borate). These C–H activated complexes underwent reductive elimination at varying temperatures (24–70 °C) in C6D6 or C6D12. Using previously established kinetic techniques, the relative Rh–C bond strengths were calculated. Analysis of the relative Rh–C bond strengths vs. C–H bond strengths shows a linear correlation with slope RM–C/C–H = 1.22 (12). In general, α-substituents increase the relative Rh–C bond strengths compared to the C–H bond that is broken.
CNneopentyl), underwent oxidative addition of substituted ketones and esters resulting in Tp′Rh(CNneopentyl)(R)(H) complexes (Tp′ = tris-(3,5-dimethylpyrazolyl)borate). These C–H activated complexes underwent reductive elimination at varying temperatures (24–70 °C) in C6D6 or C6D12. Using previously established kinetic techniques, the relative Rh–C bond strengths were calculated. Analysis of the relative Rh–C bond strengths vs. C–H bond strengths shows a linear correlation with slope RM–C/C–H = 1.22 (12). In general, α-substituents increase the relative Rh–C bond strengths compared to the C–H bond that is broken.
- This article is part of the themed collection: Breaking bonds over many timescales: in celebration of Robin Perutz’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...