Photo-induced charge separation in hydroxycoumarins on TiO2 and F–TiO2†
Abstract
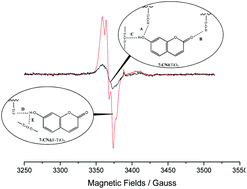
The efficiency of photocatalytic charge separation is much higher for 7-hydroxycoumarin (7-CN) and 6,7-dihydroxycoumarin (6,7-CN) adsorbed on the surface modified TiO2 where the surface hydroxyl group was replaced by a fluorine atom (F–TiO2) than on TiO2. EPR measurements find 5- and 12-fold increases in free radical yields for 7-CN and 6,7-CN, respectively. DFT calculations for the coumarins on TiO2 and F–TiO2 were performed to investigate these phenomena. The calculations show that when the coumarins act as the H-bond donors, the driving force for photo-induced electron transfer from the dyes to TiO2 is higher, and the dye's excited state mixes strongly with the TiO2 conduction band states. This is attributed to the shorter distance between the coumarins and the surface of TiO2 when the coumarins act as the H-bond donors. These calculations explain why the efficiency of charge separation of the coumarins is much higher on F–TiO2 than on TiO2.


 Please wait while we load your content...
Please wait while we load your content...