Computational prediction of pentadentate iron and cobalt complexes as a mimic of mono-iron hydrogenase for the hydrogenation of carbon dioxide to methanol†
Abstract
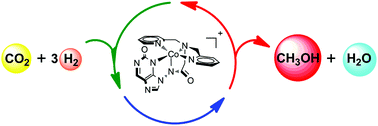
A series of amidate-ligated pentadentate iron and cobalt complexes with N-heterocyclic pyridinol groups were proposed and computationally screened as potential catalysts for CO2 reduction. Density functional theory calculations reveal a ligand assisted heterolytic H2 cleavage mechanism with a total free energy barrier of 23.3 kcal mol−1 for the hydrogenation of CO2 to methanol catalysed by a pentadentate Co complex with a 2-[bis(pyridine-2-ylmethyl)]amino-N-3,9-purin-2-one ligand.


 Please wait while we load your content...
Please wait while we load your content...